Pharmaceutical manufacturing, biotechnology, medical research, cosmetics, or electronics… A cleanroom is far more than just a workspace it’s a highly controlled ecosystem where every detail matters. With stricter ISO standards, more frequent quality audits, and growing traceability requirements, laboratories must rethink their methods. How can you ensure an impeccable level of cleanliness while optimizing performance and compliance? By embracing the innovations that are redefining ultra-clean management! Discover the top innovations for your cleanrooms.
Why is ultra-clean innovation essential in cleanrooms?

Cleanrooms lie at the heart of the most sensitive and demanding processes. Medications, electronic components, and chemical products are all manufactured there under the strictest precautions. To prevent any cross-contamination, these environments require constant particle monitoring and flawless control of hygiene and ultra-cleaning protocols. These highly protected spaces are governed by ISO 14644, which sets rigorous standards for cleanliness, environmental monitoring, and airflow management.
In recent years, events such as the COVID-19 health crisis, food contamination scandals, and industrial incidents have further tightened regulatory requirements. The traceability of ultra-cleaning operations has now become a cornerstone of quality management.
At the same time, cleanroom companies face increasing pressure: maintaining high operational performance, protecting operators, and optimizing costs. These demanding constraints make traditional management methods less and less effective.
This is where technological innovation plays a key role. The digitalization of processes, the integration of connected sensors, and the use of real-time data are transforming the management of ultra-clean operations within cleanrooms:
- Reduced contamination risk through better control of airflow and standardized procedures
- Enhanced regulatory compliance with automated, timestamped, verifiable control records accessible at any time
- Time and efficiency gains thanks to the automation of quality controls, non-conformity management, and data correlation
- Easier audit preparation through centralized, easy-to-read dashboards updated in real time
How can you digitalize your ultra-cleaning processes?
What ultra-clean standards must be met in cleanrooms?
The digitalization of ultra-cleaning processes: yes but it’s not that simple. There are several requirements to meet. Working in a cleanroom involves complying with a strict set of rules and obligations, both in the design of the spaces and in the daily practices of the teams.
Classification and Ultra-Clean standards
The ISO 14644 standard is the global benchmark for cleanroom classification. It defines air cleanliness levels based on the concentration of particles present in one cubic meter of air. Each cleanroom is classified from ISO 1 to ISO 9, depending on the maximum allowable number of particles.
The lower the class, the stricter the particle control must be: high-performance filtration systems, optimized airflow circulation, and cleaning and ultra-cleaning procedures tracked in real time.

In pharmaceutical environments, these requirements are further reinforced by Good Manufacturing Practices (GMP). This regulatory framework governs the design, operation, and maintenance of facilities where ultra-clean conditions are essential. The goal is to ensure a stable, controlled environment that complies with the sector’s quality standards.
Continuous monitoring of the environment and airflow
Maintaining environmental conditions is a cornerstone of quality, ultra-cleanliness, and hygiene in cleanrooms. Every parameter must be continuously monitored to minimize infectious or microbial risks between zones.
This monitoring relies on several key elements:
- Differential pressure: creates an air barrier between zones with different cleanliness levels. Positive pressure prevents particle intrusion, while negative pressure limits their spread.
- Temperature and humidity: maintaining stable levels prevents microbial growth and ensures a safe working environment.
- Laminar airflow: provides constant particle sweeping and keeps critical areas in optimal conditions.
- Air exchange rate: adjusted according to the targeted ISO class, it helps maintain a pure atmosphere even during high activity.
Strictly regulated hygiene and ultra-cleaning protocols
In a cleanroom, nothing is left to chance. Every action, every product, and every intervention is recorded in strict operational protocols. Cleanroom ultra-cleaning procedures list, step by step, the approved products, intervention frequencies, application methods, required actions, and task validation procedures.
Traceability plays a key role in these processes. In addition to complying with current protocols, every operation must be verifiable, controllable, and auditable at any time. This level of rigor not only ensures the safety of both procedures and operators but also demonstrates full control during inspections.
Top 5 ultra-clean innovations for your cleanrooms
In an environment where the slightest particle can compromise an analysis or production, ultra-clean technologies are redefining standards.
AI, automation, connected sensors… increasingly smart solutions are emerging to ensure perfect control of critical environments. Here are the 5 ultra-clean innovations that will permanently transform the management of your cleanrooms:
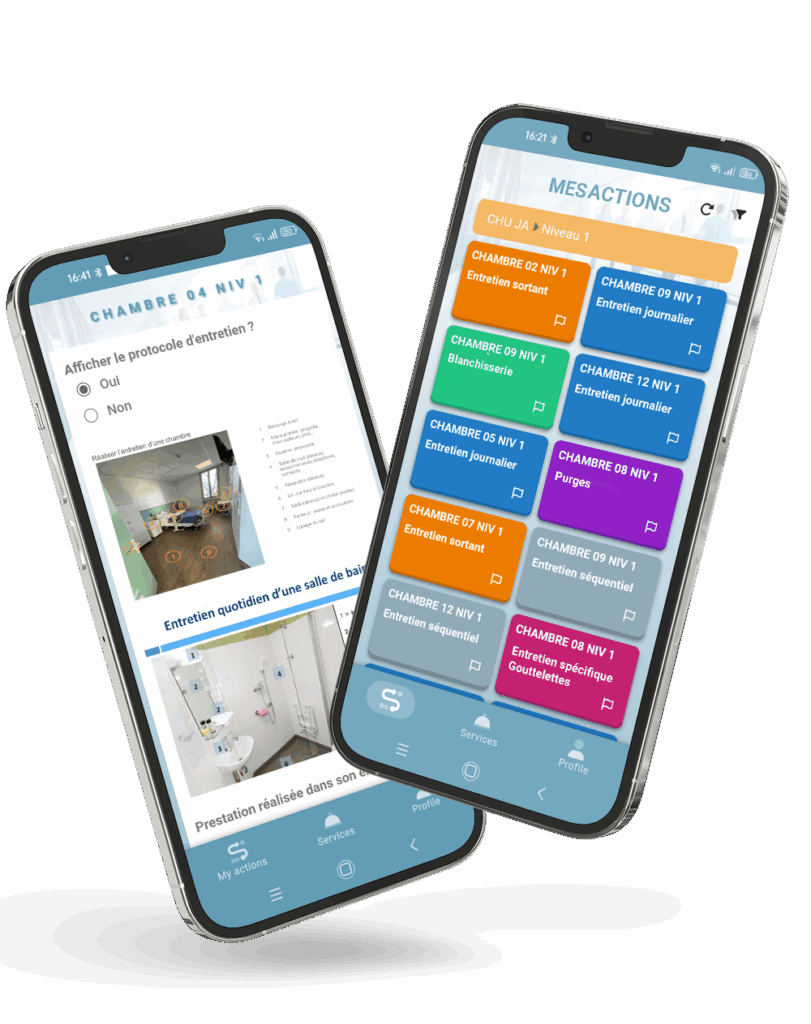
1. Digital audit applications
Faced with strict traceability and compliance requirements, cleanrooms must be able to prove the quality of their cleaning and ultra-cleaning protocols at any time. Beyond task monitoring, ensuring the reliability of records is also mandatory. The international standard 21 CFR Part 11 defines these security requirements every action must be authenticated, timestamped, and protected for ultra-cleaning audits.
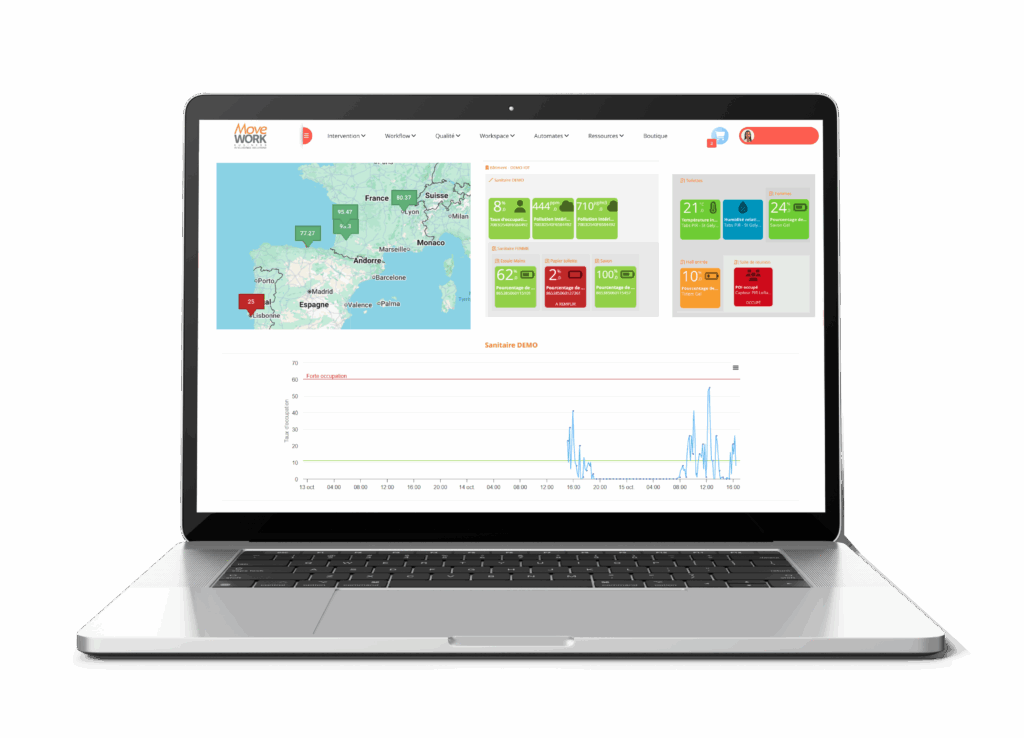
With the myMissions Indoor mobile app and the Ultra-Cleaning add-on, MoveWORK provides a comprehensive solution to meet these obligations:
- Illustrated protocols updated in real time
- Cleanroom mapping by zone or class
- Tamper-proof audit trails and timestamped photo evidence
- Monitoring of gaps between planned and completed tasks
- Automatic measurement of acceptability thresholds and corrective plan automation
- Automatic data history, encryption, and backups
Everything is designed to ensure full compliance of your ultra-cleaning processes. This digitalization secures every step of cleanroom interventions and guarantees total transparency during quality controls. Managers benefit from a clear, real-time view of all operations.
Successful audits, guaranteed?
2. Smart sensors and IoT
Thanks to IoT, connected sensors are redefining continuous monitoring of cleanroom environmental conditions. Temperature, humidity, differential pressure, fine particles every parameter can now be automatically measured, analyzed, and modeled. Connected to a centralized platform like MoveWORK Flow,
these IoT sensors collect and transmit real-time data, which is then measured and compared against acceptance thresholds. This intelligent supervision strengthens flow control and paves the way for high-performance predictive maintenance.
Solutions such as the Nexelec Sign sensor perfectly illustrate these technological advancements. This sensor simultaneously measures CO₂, temperature, humidity, light, noise, and human presence, providing a comprehensive view of indoor air quality and safety.
3. The robotization and automation of ultra-cleaning
Cleaning robots and cobotics are now making their way into ultra-clean environments. Using advanced 2D or 3D spatial modeling systems, they can perform repetitive tasks with exceptional precision. In practice, these machines now integrate several major innovations:
- Space modeling and autonomous navigation within cleanrooms between multiple treatment stations
- Use of materials designed to minimize particle emissions, outgassing (ammonia, hydrocarbons), and electrostatic discharge
- Fleet management to trigger routes, optimize downtime, and distribute tasks among multiple machines
- Task flexibility and adaptation to variable work rates
In short: in ultra-clean environments, these robots are no longer just automated “floor cleaners.” They combine intelligent control and operational versatility to meet the highest ultra-cleaning standards.
4. Connected and traceable consumables
The tracking of consumables is entering a new era thanks to digitalization. Is a disinfectant dispenser running low? Replenishment is triggered automatically. Connected technologies now allow continuous monitoring of stock levels and equipment, preventing shortages and reducing contamination risks in cleanrooms.
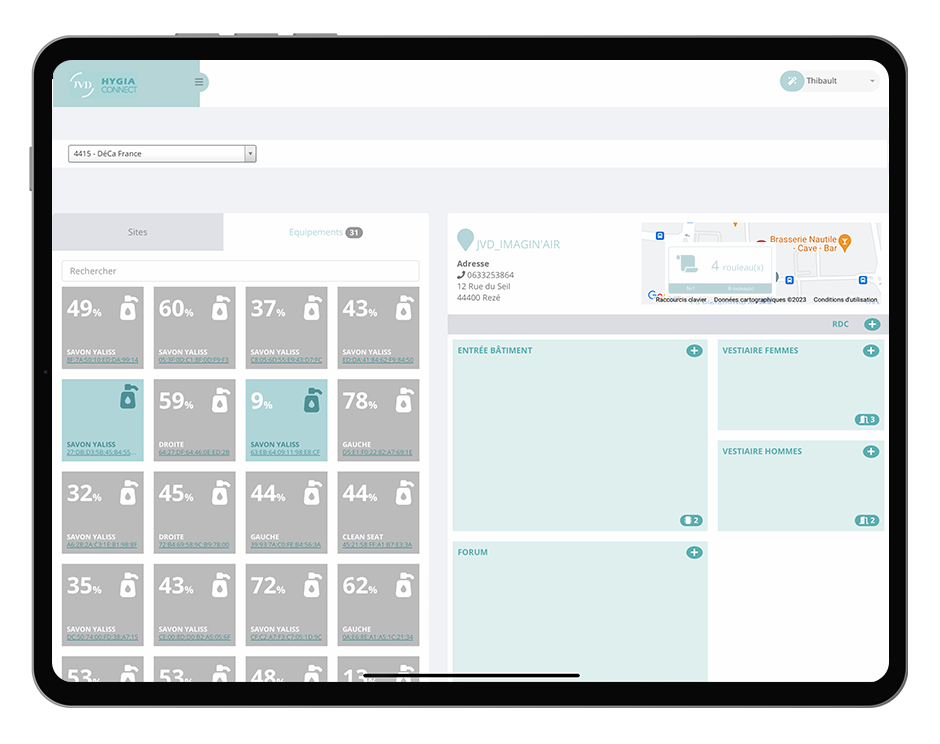
JVD has developed HygiaConnect, an innovative solution capable of tracking refill levels, usage frequencies, and maintenance alerts in real time. Connected to the MoveWORK Flow platform, these devices provide complete traceability for your cleanroom ultra-cleaning operations. Every level of product usage is recorded, ensuring safety thresholds are always maintained.
Beyond simple logistical tracking, this digitalization improves the allocation of operational, material, and human resources along the production workflow. This optimized management also directly contributes to reducing operating costs.
5. Digitalization of ultra-cleaning operations management
Gone are the days of paper schedules, logbooks, or manual timecards! Biometric systems, AI, smart QR codes, infrared, NFC… With platforms like MoveWORK Flow, the management of ultra-cleaning and maintenance operations enters a new dimension: centralized, automated, and fully traceable.
From a single interface, every intervention is planned, executed, monitored, and validated in real time. Field teams have access to clear and
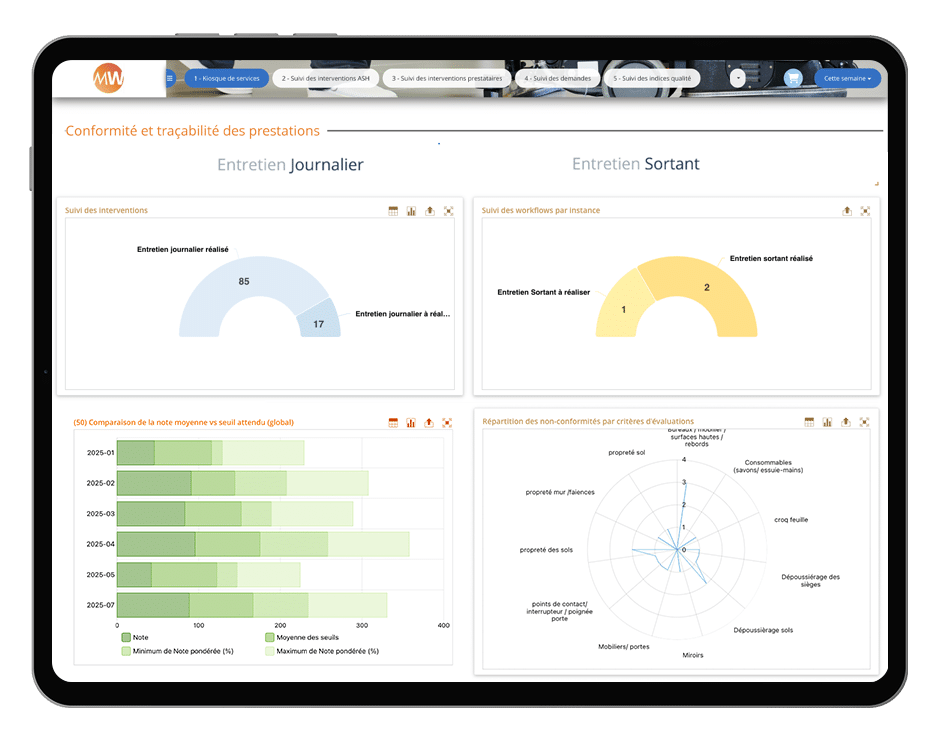
up-to-date instructions, while quality managers gain a comprehensive, real-time view of all activities: completed tasks, adherence to frequencies, observed deviations, compliance rates, and corrective actions.
This operational transparency ensures full compliance with ISO and GMP requirements. By eliminating repetitive administrative tasks, site managers save valuable time that can be reinvested in strategic oversight and the continuous improvement of ultra-cleaning processes in cleanrooms.