In critical environments such as hospitals, pharmaceutical laboratories, cleanrooms, or food production facilities, the slightest hygiene failure can have major consequences. Every action, every movement, every step matters. The rigor of protocols is what ensures patient safety, product quality, and partner trust. From applying strict procedures to control contamination risks to ensuring compliance with the highest standards, ultra-cleanliness is what defines this absolute level of precision and safety.
However, many organizations still rely on manual monitoring methods: paper sheets, Excel files, time-consuming checklists, and evidence that is hard to verify during audits. These outdated practices hinder traceability and slow down access to the certifications that are essential in ultra-clean sectors.
In this article, discover our expert advice to help you successfully digitize your ultra-cleanliness processes!
Why digitize ultra-cleanliness processes?
Ultra-cleanliness requires an exceptionally strict level of control: monitoring airborne particles, managing microorganisms on surfaces, ensuring floor cleanliness, and validating every protocol applied. These demands directly concern critical environments such as hospitals, cleanrooms, the aerospace and aeronautics industries, or the agri-food sector—where even the slightest deviation can have serious consequences.
To reduce the risk of non-compliance with ultra-cleanliness protocols
When ultra-cleanliness protocols are not followed, companies responsible for these sensitive areas face significant risks:
- Nosocomial infections or large-scale contamination
- Deterioration of product quality
- Regulatory non-compliance leading to failed audits, sanctions, or loss of certifications
- Reputational and financial risk due to loss of client or partner trust
- Destruction of entire batches in the event of confirmed contamination
- Production shutdown leading to plant closure
- Health crisis and potential fatalities

So how can you avoid ending up in these situations? By digitizing your ultra-cleanliness processes. By replacing manual methods with digital tools, you ensure complete traceability of every intervention. And you can go even further! You can automate the entire control chain, simplify audits, enhance your teams’ responsiveness, and guarantee continuous compliance across all your critical environments.
To ensure compliance with regulatory standards and certifications
To guarantee the efficiency of your ultra-cleanliness operations, it is essential to comply with a set of legal, structural, and operational requirements. Ultra-cleanliness goes far beyond a voluntary approach, it is strictly regulated by European and international standards. These frameworks define the criteria to be met and the acceptable thresholds for each hygiene procedure, ensuring the safety, quality, and traceability of all interventions.
Here are the main international standards governing Ultra-Cleanliness:
- ISO 9001 – Quality Management and Continuous Improvement: Provides a framework for implementing a documented quality management system, with monitoring of deviations, corrective actions, and continuous improvement.
- ISO 14644 – Air Cleanliness and Particle Control: Defines particulate and microbiological cleanliness classes in cleanrooms, as well as measurement and control methods.
- GMP – Good Manufacturing Practices: Imposes strict control over sterile production environments, particularly in laboratories and hospital pharmacies.
- 21 CFR Part 11 – Electronic Records and Signatures: Regulates the use of electronic records and signatures, ensuring their authenticity and traceability.
These standards demonstrate that an organization applies reliable protocols and secures its operations in the most sensitive environments. But how can you simultaneously monitor and secure dozens of checklists, each containing hundreds of parameters? Without a digital system or an ultra-cleanliness traceability application, this task is nearly impossible!
Ultra-Cleanliness Audit
To ensure the safety of patients, consumers, and users
Failure to comply with ultra-cleaning protocols directly exposes populations to the risk of massive contamination. Air, surfaces, medical equipment, and floors can all become carriers of dangerous microorganisms. And the consequences go far beyond simple complications, they can endanger the lives of infected individuals.
In this context, cleanliness, disinfection, and microbiological and particulate controls form essential protective barriers. This is why the traceability and reliability of protocols are crucial. However, the larger your operations, the more areas there are to monitor and the greater the risk of human error. Despite your best efforts, you simply can’t have eyes and ears everywhere. That’s where digitalization, through an ultra-cleaning management software, becomes your greatest ally.
Preserve the image and credibility of establishments
In critical environments, a single failure can be enough to permanently damage an establishment’s reputation. An unfavorable audit, a health incident, or a publicized non-compliance can undermine the trust of patients, clients, or partners. And in sectors where safety and quality are core values, regaining that credibility can take years.
The digitalization of ultra-clean processes helps strengthen transparency and prove compliance at any time. With timestamped data, automated reports, and traceable evidence, you demonstrate your rigor and reliability. The result: a stronger reputation, enhanced trust relationships, and greater competitiveness in your market.
What are the operational constraints of ultra-cleanliness?

Ultra-cleanliness imposes a highly demanding framework where nothing can be left to chance. Ensuring an environment completely free of contaminants, particles, and microorganisms is a delicate mission. These constraints include:
- Compliance with strict standards and reference frameworks
- Encryption and timestamping of all data flows and actions
- Trained, authorized, and properly equipped personnel following rigorous gowning protocols
- Use of dedicated equipment and consumables
- Zoned areas with strictly controlled circulation flows
- Cleaning and disinfection procedures that must be followed to the letter
- Frequent quality controls are essential to validate compliance
- Continuous risk management
- Comprehensive monitoring and traceability of all activities with a tamper-proof audit trail
Faced with these constraints, digitalization has become an essential lever. Your teams cannot manage all these requirements manually! Ultra-cleanliness software solutions automate monitoring, centralize data, ensure reliable traceability, and provide a clear, real-time view of operations shared by all stakeholders.
How to digitalize your ultra-cleanliness processes?
You’ve got it rigor and precision are the cornerstones of ultra-cleanliness. When managed through manual methods, it opens the door to human errors and complex monitoring. Digitalizing ultra-cleanliness processes strengthens safety by minimizing oversights. But concretely, what are the key steps to digitalize your ultra-cleanliness processes in critical areas? Discover them below!
1. Assess the current situation
It all begins with a precise and objective assessment of your procedures and your production or intervention processes. It’s essential to analyze your current practices by observing how schedules are organized, how interventions are monitored, how quality controls are carried out, and how documentation is managed and stored. Who, what, where, how, and why? Each parameter must be examined from every possible angle.
This analysis will highlight the points that slow down your teams, the time-consuming manual tasks that weigh on daily operations, and the sensitive areas that require reinforced monitoring and flawless traceability. This diagnostic phase forms a solid foundation and helps you clearly identify your real challenges.
Pro tip: Draft a comprehensive specification document listing all the requirements from the ultra-cleanliness reference standards. Once completed, directly compare your interventions with the established needs.
2. Define Your Needs
Before starting the digitalization process, you need to take a step back and analyze your on-the-ground realities. Identify your needs: do you need to strengthen the traceability of cleaning operations in your critical areas? Demonstrate compliance with quality standards? Optimize team scheduling? Or access real-time indicators to respond more quickly in case of non-compliance? This step allows you to prioritize your objectives and target the levers that will have the greatest impact.
This clear vision ensures that your digital transformation is not just a technological project but a strategic response aligned with your operational and regulatory requirements.
3. Choose the right ultra-cleanliness software
There are many options available on the market, but not all of them meet the same level of requirements. To ensure maximum performance, your ultra-cleanliness solution must above all:
- Ensure regulatory compliance by integrating applicable standards and easily generating proof during audits
- Guarantee full, real-time traceability with timestamped and secured tracking of every action
- Offer maximum data reliability centralized, tamper-proof, and accessible at any time for inspections
- Be simple and user-friendly, so that all profiles can easily use it from a smartphone or tablet
- Integrate seamlessly into your existing ecosystem, connecting with your IoT sensors and other systems
- Remain flexible and scalable to adapt to your sites, protocols, and future evolutions
- Facilitate management and analysis through real-time dashboards and automatic alerts in case of deviations or non-conformities
- Come with strong support services, including training and assistance when needed
Start by conducting active research on the solutions available on the market. By comparing existing tools and their ability to integrate into your critical environments, you can concretely assess which solutions meet your operational constraints and which ones might slow down your project.
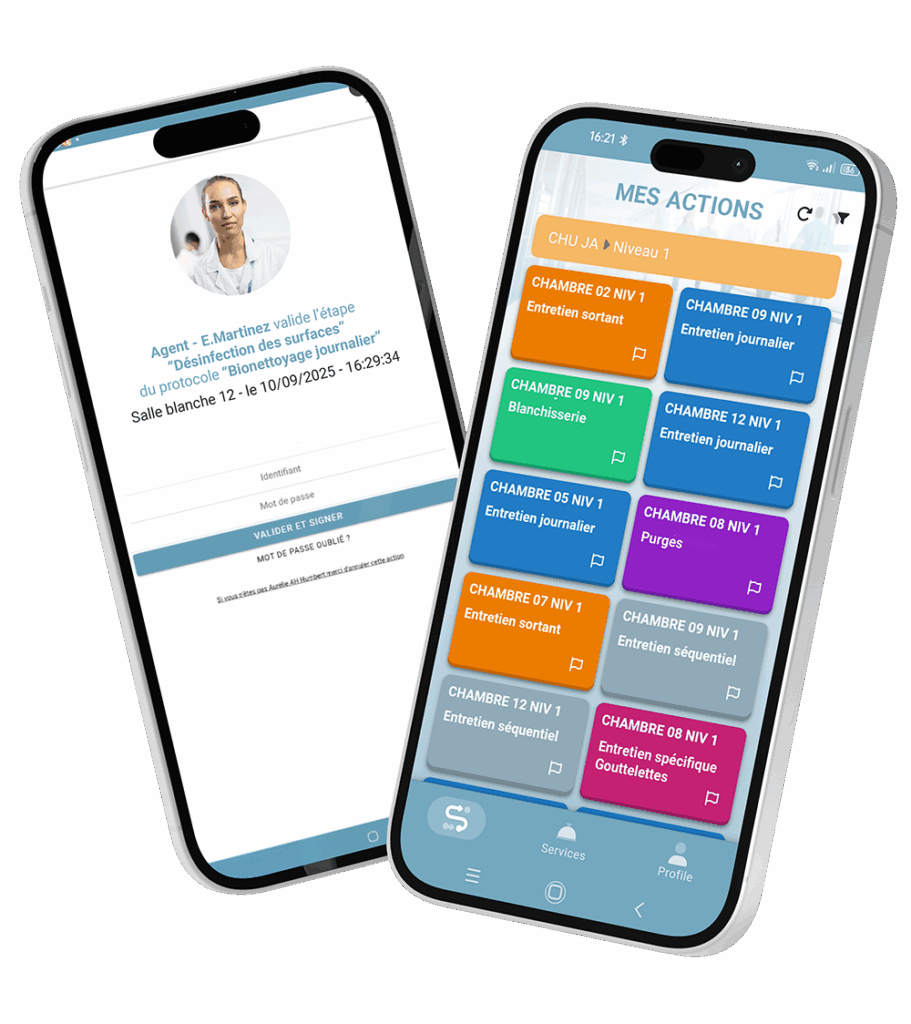
The new Ultra-Cleanliness add-on of MoveWORK Flow has been designed for environments where errors are not an option. It integrates advanced features that ensure total control over your operations. Its compliance with 21 CFR Part 11 meets the strictest standards, while the tamper-proof, timestamped, and certified audit trail guarantees flawless traceability. Electronic signatures secure every critical step, and mandatory human validation on critical workflows reinforces process reliability. The designation of authorized personnel whether contractors or clients frames each action and ensures accountability for all involved parties. Quality controls rely on digitalized checklists, photographic evidence, and on-site validations, which can be complemented by a biometric terminal for an enhanced level of security and authentication.
Beyond compliance, the benefits are tangible and immediate: stronger differentiation from generic solutions, reduced risk of penalties or quality issues, easier maintenance of your ISO certifications, and long-term protection of your contracts and margins. In practical terms, this means less time wasted managing evidence manually, real-time visibility over operations, and enhanced trust among all stakeholders. For quality managers, it ensures reliable performance indicators. For teams, it provides a simple tool that guides them in their daily tasks. And for management, it enables more transparent and confident decision-making.
4. Train your employees
In ultra-clean environments, technology alone is not enough human expertise lies at the heart of the system. Agents operating in critical areas must be trained and certified in ultra-clean procedures before they can even begin working. Training your teams empowers them to understand why this change is being made and how it simplifies their daily tasks. The more they recognize the direct benefits for their work, the more naturally they embrace the tool.
In addition, there is an essential requirement: mandatory training and certifications specific to each industry. Whether it’s HACCP in the food sector, hospital hygiene protocols in healthcare, GMP in the pharmaceutical industry, or electrical safety certifications in the energy field, your employees must hold the appropriate qualifications to operate.
5. Deploy the solution
Finally comes the moment to deploy the chosen solution. Deploying an Ultra-Cleaning solution is far more than a simple technical installation. The technology must be embedded into your operational practices and adapted to your specific constraints.
In practice, deployment begins with a pilot phase on a limited scope a cleanroom, a food production line, or a hospital ward. This real-life test allows you to evaluate configuration relevance, measure team adoption, and fine-tune workflows according to your specific needs. Once validated, the solution is gradually extended to all critical areas and integrated into your existing systems. This progressive rollout secures the transition and minimizes the risk of disruption in daily operations.
At MoveWORK, our expert consultants support you throughout the entire process. Their role goes far beyond technical implementation: they analyze your needs, translate regulatory requirements into operational configurations, and ensure your teams fully adopt the tool. Thanks to their industry expertise, they guarantee a tailored and efficient deployment.